Adsorption: How do binders act – the Bentonite example
Adsorption (or biosorption) is a fascinating process where mycotoxins interact with binders on their surface or within their layers. This interaction occurs through non-covalent bonds, such as hydrogen bonds, Van der Waals forces, ionic bonds, and hydrophobic interactions.
What are non-covalent bonds?
Non-covalent bonds are a type of chemical interaction that occurs between atoms or molecules without the sharing or transfer of electrons. There are several types:
- Ionic bonds: Ionic bonds are formed between ions of opposite charges. One atom donates electrons (cation), while another atom accepts electrons (anion).
- Hydrogen bonds: Occur when a hydrogen atom is covalently bonded between two electronegative atoms (such as oxygen or nitrogen.
- Van der Waals forces: arise from temporary fluctuations in electron distribution within molecules. These forces can be categorized into three types: London dispersion forces, dipole-dipole interactions, and dipole-induced dipole interactions. London dispersion forces occur between all molecules. (Figure 1)
- Hydrophobic interactions: occur between nonpolar molecules in the presence of water. Nonpolar molecules are hydrophobic (water-repellent) and tend to aggregate together to minimize contact with water molecules.(Figure 2)
Figure 1: Van der Waals forces

Figure 2: Hydrophobic interactions

Most mycotoxins are polar like Aflatoxin, DON, T2 – HT2, Orchatoxin A, Fumonisin whilst ZEA is non polar.
How does bentonite bind to polar mycotoxins like aflatoxin?
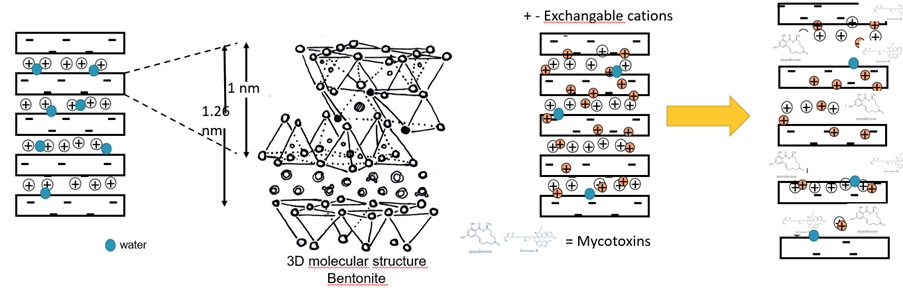
Bentonite has a layered structure composed of negatively charged sheets of silicate minerals. These negatively charged surfaces can attract and bind positively charged ions or molecules through ionic interactions. (Figure 3)
Figure 3: Mode of action of bentonite binding to polar mycotoxins (AFLA)
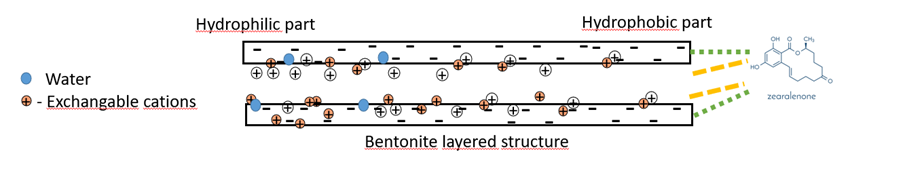
How does bentonite bind to non-polar mycotoxins like ZEA?
In the case of non-polar mycotoxins, which lack a charge, the binding is primarily driven by hydrophobic interactions and van der Waals forces.
The hydrophobic regions of the mycotoxins interact with the hydrophobic surfaces of the bentonite. This hydrophobic interaction allows the non-polar mycotoxins to adsorb onto the surface of the bentonite particles.
The van der Waals forces further stabilize the binding by enhancing the attractive interactions between the mycotoxins and the clay surfaces.
Figure 4: Mode of action of bentonite to non-polar mycotoxins (ZEA)
In summary, adsorption (or biosorption) is a crucial process where mycotoxins interact with binders through non-covalent bonds, including hydrogen bonds, Van der Waals forces, ionic bonds, and hydrophobic interactions. Understanding these non-covalent bonds helps us appreciate how substances like bentonite can effectively bind both polar and non-polar mycotoxins.
For polar mycotoxins such as aflatoxin, bentonite's negatively charged surfaces attract and bind these toxins through ionic interactions. In contrast, non-polar mycotoxins like ZEA are captured primarily through hydrophobic interactions and Van der Waals forces, ensuring a stable and effective binding.
This knowledge is essential for developing better strategies to mitigate the harmful effects of mycotoxins in various environments, contributing to safer and healthier outcomes.
Контакты:
Свяжитесь с нами, используя данную форму.







